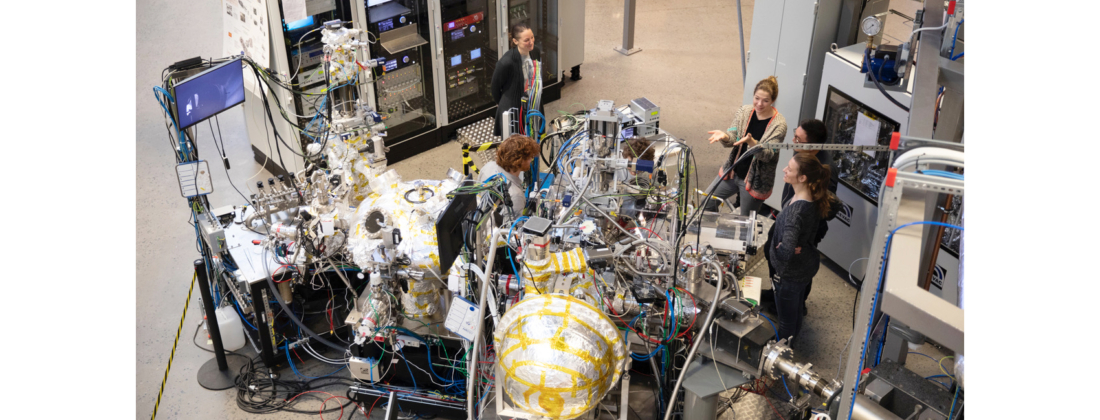
To understand the electrochemical potential of lithium-ion batteries, it’s important to decipher the chemical processes at electrode interfaces occurring during device activity. Using HIPPIE beamline, a research group investigated and modelled the influence of electrochemical potential differences in operando in these batteries.
“With our experiments at HIPPIE, we had the opportunity to look at battery materials and interface reactions under operating conditions exploring the capabilities of the electrochemical setup at the end station,” said Julia Maibach, study author and professor at the Institute for Applied Materials – Energy Storage Systems at Karlsruhe Institute of Technology (KIT) in Germany. “We were among the first users testing the electrochemical set up including the glove box for inert sample transfer.”
Why study electrochemical potential difference in batteries? This phenomenon drives the transfer of charged particles to different phases in redox reactions at battery electrode-electrolyte interfaces. In simple terms, the difference enables the chemical reaction necessary for Li-ion battery function.
A central question the researchers considered—how does the electrochemical potential difference affect redox reactions on the solid-liquid interfaces during Li-ion battery operation? Probing gold and copper model electrodes with ambient pressure photoelectron spectroscopy (APPES) during lithiation (charge transfer), the team was able to follow changes in the electrochemical potential over these interfaces by measuring the kinetic energy shifts of the electrolyte levels, which could be correlated to the electrochemical reactions occurring at the electrode/electrolyte interface. The experimental setup allowed for an indirect probe of the electrode interfaces.
The electrochemical potential difference of electrons is measured with voltage. The results showed that a change in applied voltage to a (solid) electrode interface, produced a change in the electrochemical potential of the (liquid) electrolyte.
The study follows earlier work by the group at MAX-lab in Lund to investigate battery electrolytes statically using the APPES technique at SPECIES beamline.
“There is still a lot to learn when using APPES for battery research, but every step brings us closer to follow the important interfacial reactions at the solid-liquid interface under as realistic conditions as possible,” said Maibach.
The group continues the work at HIPPIE beamline, looking at lithium titanate electrodes and different electrochemical protocols. Their results hold potential for better understanding of charge transfer at the interfaces and charge storage mechanisms in the electrode.