Recent research by a group of researchers from Sweden, the US, and the UK successfully developed a specific NUDT15 inhibitor, TH7755, that helped the group to obtain structures of clinically relevant NUDT15 variants: Arg139Cys, Arg139His, Val18Ile, and V18_V19insGlyVal. These insights allow further understandings of the structural basis of thiopurine intolerance in patients carrying these NUDT15 mutants and will support the prediction of new NUDT15 mutants’ effects in clinical treatments.
Today, clinical treatments of cancers, such as leukemia, and other inflammatory diseases, rely significantly on thiopurine drugs. The enzyme NUDT15 metabolizes these drugs. However, researchers have found that patients with mutated NUDT15 gene often tolerate these drugs poorly and suffer severe side effects during the treatment. While some studies have observed specific NUDT15 variants (e.g., R139C), others are still unknown. This has made it difficult to predict the extent of thiopurine toxicity the patients will be facing in their treatments.
‘Prior to this study, the reason why certain NUDT15 mutations cause severe toxicity was not well understood. We, therefore, became interested in determining the X-ray crystal structures of clinically relevant NUDT15 mutants to see whether thiopurine toxicity phenotypes could be explained from a structural standpoint,’ explained Emma Scaletti, a postdoc in the laboratory of Prof. Pål Stenmark who was involved in the study.
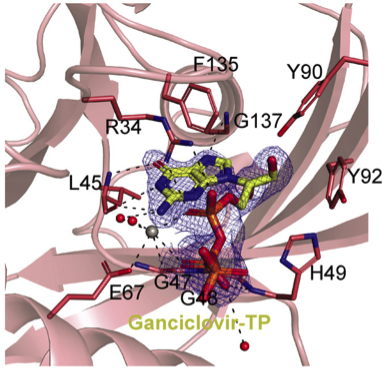
A breakthrough for NUDT15 mutants structural observation
The NUDT15 mutants’ high instability has been the biggest challenge for researchers to obtain structural information. The development of TH7755 inhibitor from this research was a breakthrough. TH7755 made it possible to obtain well-diffracting crystals of NUDT15 mutants and improved cellular target engagement and 6-thioguanine potentiation compared to the existent inhibitor, TH1760. It also showed no toxicity of its own. TH7755 stabilized the NUDT15 mutants to a great extent, enabling the analysis by X-ray crystallography.
The data collection for this research was done prior to the Covid-19 pandemic on-site at MAXIV, using the BioMAX beamline. However, members of Prof. Stenmark’s group based at both Stockholm University and Lund University currently collect all X-ray data remotely due to the ongoing Covid situation. ‘Remote data collection at MAXIV is fast and convenient. This has been especially useful during the Covid-19 pandemic’, Scaletti stated.
Scaletti is looking forward to more research to be done with the TH7755 inhibitor. ’In the future, we hope to use our newly developed inhibitor as a tool to study other clinically relevant NUDT15 mutants. We hope that this information can be used to complement pre-emptive NUDT15 genotyping to provide better treatment options and outcomes for patients,’ explained Scaletti.