To sever society’s tether from fossil fuels, the development of more efficient catalysts for renewable energy production is a recognized, key step. On surfaces covered by 2D materials, a more detailed picture of the reaction process will greatly enhance our understanding, according to a recent study in ACS Catalysis. Researchers in Sweden have observed the effects of hydrogen and other gas combinations on 2D material graphene during undercover reactions using ambient-pressure XPS at MAX IV’s HIPPIE beamline.
In the field of catalysis research, a relatively new strategy for molecular energy conversion is the use of space-confined catalysis, with undercover catalysis being one example. Scientists are learning that certain catalysts may enable greater control of reactions and perform with exceptional efficiency and potent selectivity when the reaction is carried out ‘undercover’, that is, in a confined molecular space. As with classic catalysis, greater efficiency can facilitate reactions with lower energy requirements and thereby, potentially lowered costs.
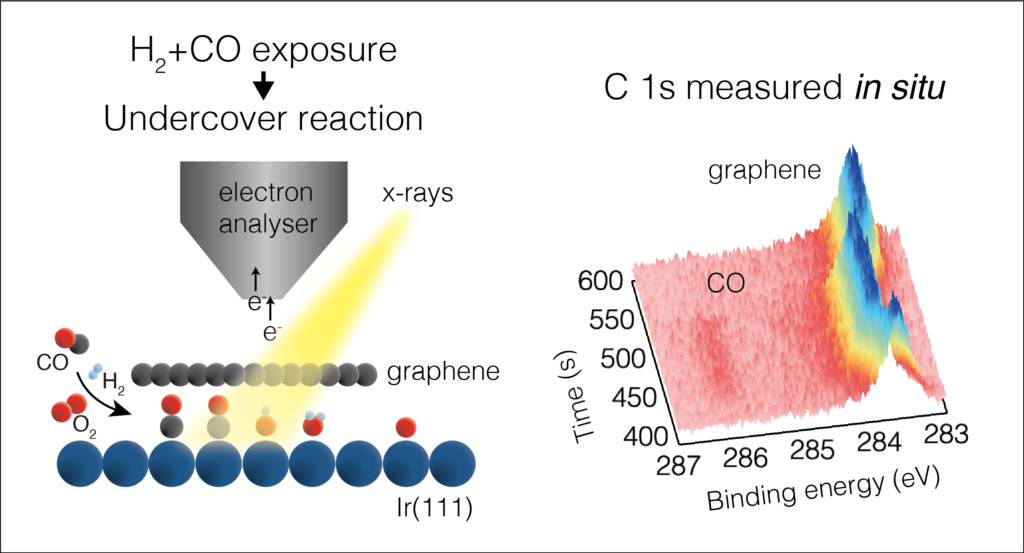
Atomic-level measurements with Ambient Pressure X-ray Photoelectron Spectroscopy (APXPS) during an evolving reaction provides knowledge of how all the elements interact. “It is very exciting that just following the spectroscopic signal from graphene we get information about the elements present underneath,” said Virginia Boix, study author and former PhD student at Lund University. “Moreover, because it’s such a strong signal, we can measure it continuously during the reaction, meaning that we can access information about the kinetics of the undercover reaction!”
Precursor for success
The research group carried out three trials of gas delivery or reactants, to a confined catalyst, iridium (substrate) placed under flakes of 2D material graphene. They sought to determine what occurs in the model system with the diffusion of gases, hydrogen, oxygen, and carbon monoxide individually and in varied combinations. An important question was, how did the gases disperse to the undercover space? This precursor step is crucial for undercover catalysts to work in a reaction, and therefore a potential roadblock for industrial application if not well understood.
“Fortunately for us this question can be addressed using the unique setup available at HIPPIE beamline,” said Boix. “The gas pulse setup at HIPPIE allows us to control which reactants get in and out the undercover space, and the high intensity [flux] allows us to follow the process in-situ, even under more realistic conditions, for example, millibar pressures.”
The group observed that the gas pulse method works controllably to intercalate (insert) and de-intercalate (remove) molecules to and from the confined space below the graphene flakes. One key finding was that hydrogen and oxygen, and the resulting undercover water formation, can promote the intercalation of larger molecules. In the future, it would be interesting to study reactions using the pulse method with such bigger, more relevant molecules, like CO2 hydrogenation for CO2 capture, explained Boix.