A research team from the Netherlands and the UK have used MAX IV to investigate a material that could make charging of lithium-ion batteries seventy times faster than today. It is a promising development for future electric vehicles and renewable energy.
Batteries have an important role to play in a sustainable society. Lightweight, fast-charging batteries open for further utilisation of electric vehicles and renewable but non-continuous energy sources, which require efficient storage to be competitive. Battery research is focused on two tracks: inventing entirely new battery technologies or further developing the lithium-ion batteries that are the most commonly used type of battery today. In the current project, the research team have used MAX IV to investigate a new electrode material for lithium-ion batteries.
“We remain interested in researching lithium-ion batteries over new technologies due to a number of factors,” says Maarten Jager, PhD candidate at the University of Groningen and one of the study’s authors.” The technology readiness level of lithium-ion batteries is very high. In the rechargeable battery market, lithium-ion batteries account for about 67% of the market share. The chemistry involved in lithium-ion batteries is also quite well-known, so there is a more straightforward path to explore new components, which could easily be implemented into the market. New technologies can often be promising, but still take years to be developed enough.”
One of the components of batteries that can be further optimised is the electrode material. The general material for the anode, the negative electrode, in lithium-ion batteries is graphite.
“Graphite has a relatively good stability, high conductivity, and low cost. However, it also has a number of major drawbacks, which reduce its performance. It has a chemistry that limits the amount of energy each unit can store and is flammable. However, its most important drawback is the amount of power it can deliver. Graphite cannot release and store energy quickly, as it would break the electrode,” says Jager. “One major threshold consumers have for choosing an electric car is the time it takes to fully charge it at a fuel station, often over 20 minutes. Significantly bringing down this charging time without compromising battery life or storage capacity is impossible with graphite. Our experiments show that by replacing graphite with copper niobate, we can, without compromising, charge the battery 70 times faster than graphite.”
The copper niobate the researchers used in their experiment is a special so-called mixed crystal phase copper niobate containing five different crystal structures. It is the first time this type of copper niobate is investigated as a battery electrode material. Generally, so-called pure phase materials containing only one crystal structure have been thought to be the best alternative for batteries, but the new results challenge this idea.
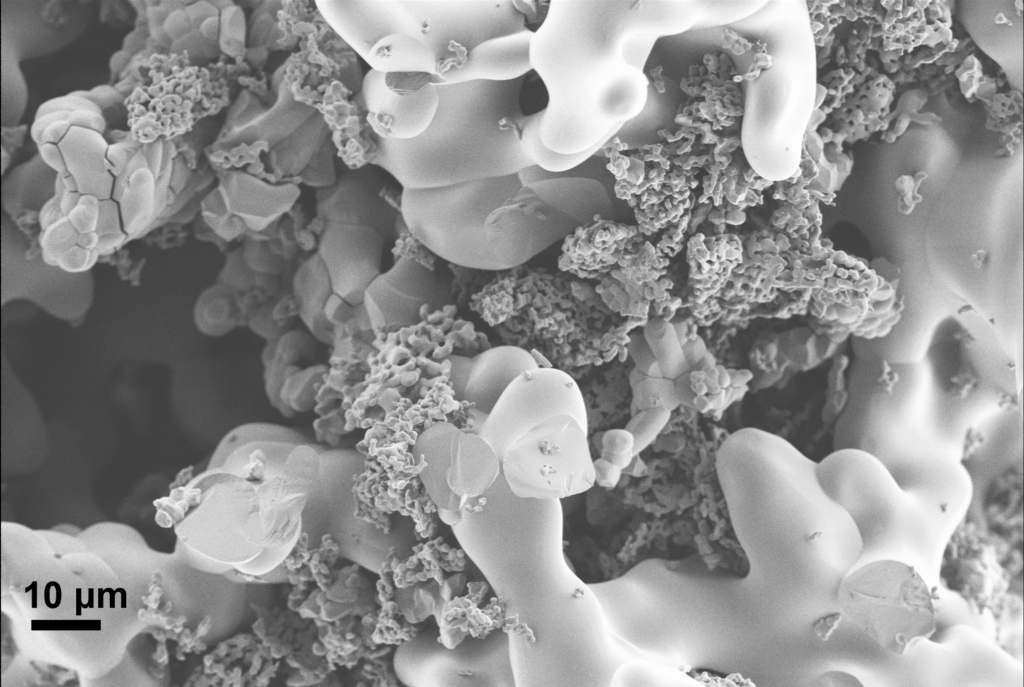
“The mixed-phase copper niobate has a higher stability, can store a lot of energy and deliver a lot of power. It can be charged and discharged an exceptional number of times over its lifetime,” says Jager.
After the copper niobate anode study, Maarten Jager expanded his focus to investigate the positive electrode, the cathode, as well.
“Cobalt is used as a part of the cathode, which is unwanted due to its ethical implications. During my current research, I am trying to understand the exact role of cobalt and whether we can replace cobalt with another material or simply increase the nickel content in the cathode to the same end. I plan to also conduct these experiments at a synchrotron,” says Jager.




