In a study conducted at MAX IV and other European synchrotrons, researchers from the Netherlands and Belgium show that the catalytic activity of highly distributed palladium depends on the size of the cerium dioxide support particles. Optimising particle size can lead to a more effective conversion of toxic carbon monoxide exhaust even in challenging cold start conditions. The study was published in the journal SCIENCE.
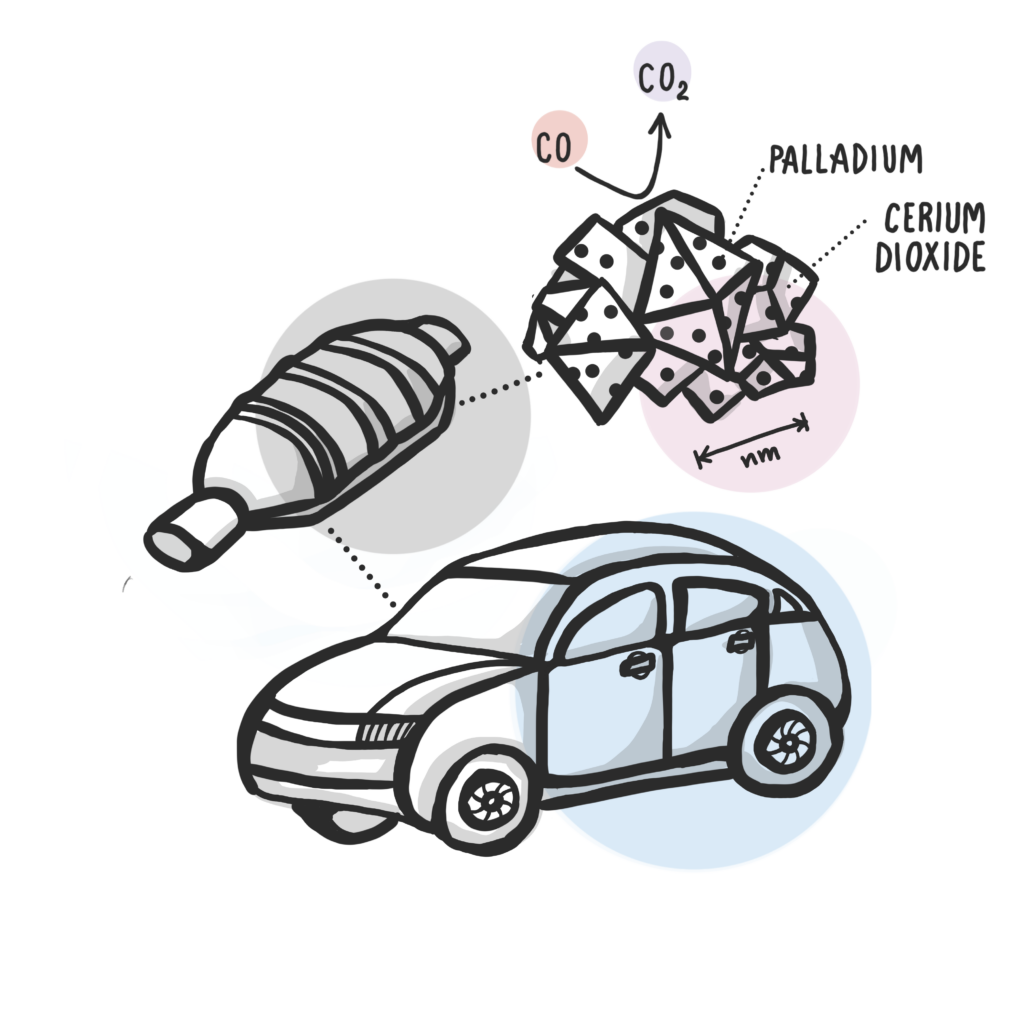
Noble metals, such as palladium, used in catalytic converters are expensive. Therefore, dispersing small amounts of the metal on top of cheaper support particles is a cost-effective way of cleaning vehicle exhaust. In state-of-the-art catalytic processes, only single noble metal atoms are used on the support particle surface. However, the size effect of the support particles has previously been unexplored for such atomically dispersed noble metal catalysts.
The catalytic activity for the palladium/cerium dioxide particles depends on how available oxygen is on the surface to react with carbon monoxide and form less toxic carbon dioxide and whether the palladium will stay distributed over the surface. Smaller support particles with a diameter of around 4 nanometres have higher activity for more carbon monoxide-rich conditions, whereas medium particles of 8 nanometres work best for the lowest temperatures.
”After studying the effect of the size of supported metal particles for a long time, it was astonishing how large the influence of the support itself on the catalytic performance of a supported metal phase is. Reducing the ceria particle size from 13 nm to below 5 nm completely changes the reaction kinetics of CO oxidation catalysed by a palladium single atom,” says Prof. Emiel J.M. Hensen, one of the researchers conducting the study.
The result is substantial in optimising catalytic converters for cold start conditions where they can efficiently convert the toxic carbon monoxide in the exhaust even before the engine becomes warm. Metals supported on cerium dioxide nanoparticles are also common in other industrial catalytic processes, such as the water-gas shift reaction for producing hydrogen, which could benefit from the results of this study.
Publication
V. Muravev, A. Parastayev, Y. van der Bosch, B. Ligt, N. Claes, S. Bals, N. Kosinov, and E. J. M. Hensen, Size of cerium dioxide support nanocrystals dictates reactivity of highly dispersed palladium catalysts, SCIENCE 380, p. 1174 (2023), DOI: 10.1126/science.adf9082