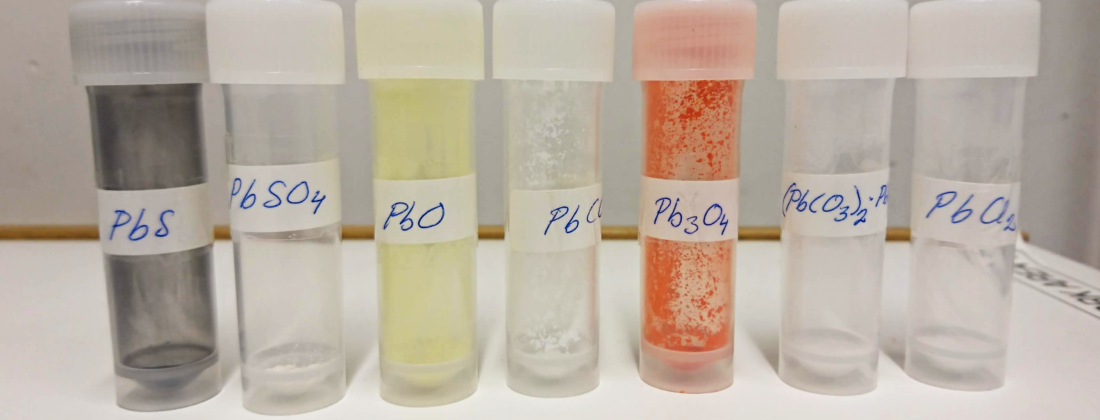
Fortum Waste Solutions, Sysav, Eon, Stena and NOAH, in collaboration with Researchers from RISE and Chalmers, used beamline Balder to identify chemical species of copper and zinc in ashes that remain after burning solid waste. Not all forms of the metals in ashes pose the same risk to the environment. Therefore, more detailed knowledge can increase the possible uses of the ashes.
Metal, plastic, cardboard, we recycle a lot of our waste. But that extra bag with the garbage does not belong in any of those categories. What about that one? Its content is often burnt to generate electricity and heat. It doesn’t, however, disappear totally, we’re left with the ashes. The chemical content of the ashes plays a significant role in how they can be used or disposed of. In this study, the researchers analysed the content of copper and zinc. Lead was also analysed but not discussed in this paper.

It all depends on what other elements the metals are bound to; they are said to exist in various species. These species have different properties when it, for example, comes to water solubility and toxic effects. It means different levels of threat to the environment and that the ashes could be used for more beneficial applications than landfills. Then we need a more detailed picture of what species they contain.
“Energy recycling from waste creates more than 1 million tons of ash in Sweden annually. The ash contains traces of toxic metals. Depending on the level and the chemical form of these metals they pose various environmental risks,” says Jenny Rissler, Lund University and RISE. “Today, a worst-case approach is taken. If we know the form of these metals, the ash may be used as a secondary raw material, replacing virgin material in-ground construction work, and does not need to be placed at landfills. Traditional methods such as X-ray Diffraction currently used for analysing chemical speciation are not sufficient at these low concentrations (<2 %) due to the complex ash matrix.”
XAS is a technique available mainly at synchrotrons, and the intense light at MAX IV allows analysing even these small amounts of metals.

“The high intensity of the Balder beamline is critical for the experiment since the sample concentration is so low,” continues Rissler. In the future, we would also like to try X-ray Emission Spectroscopy at Balder, which might give us even more details. One of the biggest challenges is the heterogeneity of the material. You have to select a small representative sample and get good spectra despite the heterogeneity.”
The metal species present in the ash depends on the specific plant and processing. Understanding this is what that the research team is focusing on next.
“There are lots of things to do, Rissler concludes. We want to learn more about other ash and the diversity of the ash coming from various waste incineration plants. The various conditions are interesting, and we also want to discover more about the stabilisation process and the physical distribution of the metals in the ash particles. We also want to continue developing the reference library at Balder to get more relevant references for waste incineration.”